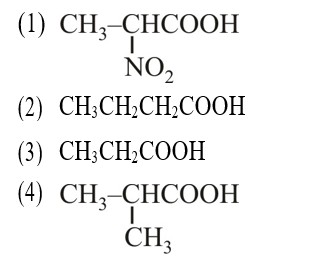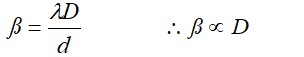On the basis of chemical reactions involved ,explain how chlorofluorocarbons cause thinning of the ozone layer in the stratosphere.
On the basis of chemical reactions involved ,explain how chlorofluorocarbons cause thinning of the ozone layer in the stratosphere.
-
1 Answer
-
This is a Short Answers Type Questions as classified in NCERT Exemplar
In the stratosphere, chlorofluorocarbons get broken down by powerful UV radiations, releasing chlorine free radical. This can be shown as-
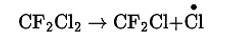
This chlorine radical then reacts with stratospheric ozone to form chlorine monoxide radicals and molecular oxygen. The reaction involved is-
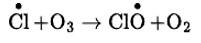
Reaction of chlorine monoxide radical with atomic oxygen produces more chlorine radicals and can be shown as-
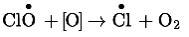
This shows that chlorine radicals are continuously regenerated and cause the breakdown of ozone. Hence, CFCs are transporting
Similar Questions for you
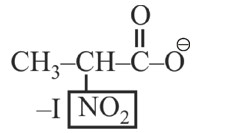
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers