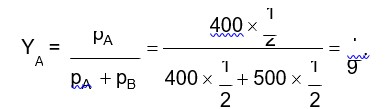Q 2.17 The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressure of 1 molal solution of a non-volatile solute in it.
Q 2.17 The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressure of 1 molal solution of a non-volatile solute in it.
Given: 1 molal solution means 1 mole of solute present in 1000g of water solvent)
Molecular weight of water = H2O = 1 * 2 + 16 = 18g/mol
No. of moles of water, n = given mass /molecular weight
⇒ n = 1000/18 = 55.56 gmol-1
Mole fraction of solute in solution, x2 = moles of solute/ (moles of solute + mole
Similar Questions for you
After the 2023-24 revised CBSE syllabus, many chapters have been removed, and in some chapters syllabus is reduced. As per the latest CBSE exam pattern 2025-26, the Chapter 1 Solutions will be asked for 5-7 marks in the Class 12 Chemistry CBSE board exams.
For other competitive exams, the Solutions c
NCERT offers the foundation for every class 12 student. CBSE board exam questions are primarily based on the NCERT exercise, Examples and conceptual explanation.
There are various benefits of using NCERT Solutions.
- Specially for CBSE Board students, these NCERT Solutions are the best resource to
Shiksha focuses on completely fulfilling the needs of CBSE class 12 board students. We provide NCERT Solutions for classes 11th and 12th in class for physics, chemistry and mathematics.
Here are the key reasons why you should use Shiksha's NCERT Solutions for the Solution Chapter:
- Complete cover
If a solution containing more than 0.9% salt is used, and we place blood cells in it, the water will flow out of the cells, and they will shrink. Such a solution is a hypertonic solution.
Similarly, if a solution contains less than 0.9% salt, the water will flow into the cells, and they will enlarge.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering