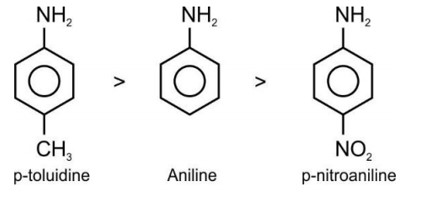
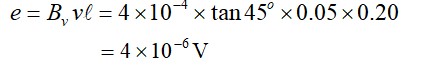The correct order of basic strength is
The correct order of basic strength is
Option 1 - <p>Aniline > p-nitroaniline > p-toluidine</p>
Option 2 - <p>p-nitroaniline > Aniline > p-toluidine</p>
Option 3 - <p>p-toluidine > Aniline > p-nitroaniline</p>
Option 4 - <p>p-toluidine > p-nitroaniline > Aniline</p>
1 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Correct Option - 3
Detailed Solution:
Electron releasing group present at para position increases basic strength.
Therefore, Correct order of basic strength is
Similar Questions for you
The direction of induced emf is to oppose changing flux.
From BF3 to BI3 Lewis acidic strength increases
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering