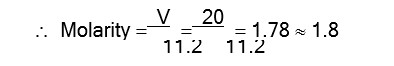The set of elements that differ in mutual relationship from those of the other sets is:
The set of elements that differ in mutual relationship from those of the other sets is:
A diagonal relationship is observed in the periodic table between elements of period (2) and period (3).
Li and Mg
Be and Al
B and Si
Li and Na do not show a diagonal relationship.
Similar Questions for you
H2S has minimum boiling point.
PbS + 4H2O2->PbSO4 + 4H2O.
Volume strength of H2O2 = Molarity * 11.2
HF molecules are associated with strong intermolecular hydrogen bonding hence its boiling point is the highest
Compound Melting Point (K)
HF 190
HCl
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering