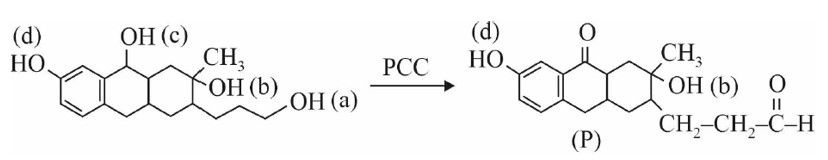
The spin-only magnetic moment value of species is________* 10-2 BM. (Nearest integer)
[Given:
The spin-only magnetic moment value of species is________* 10-2 BM. (Nearest integer)
[Given:
2 Views|Posted 6 months ago
Asked by Shiksha User
1 Answer
A
Answered by
6 months ago
Here, number of unpaired electrons, n = 1
Spin only moment ;
= 173 * 10-2 B.M
Similar Questions for you
Kindly go through the answers
(7.00)
Kindly consider the following Image
In 4d orbital, n = 4 and
Radial nodes =
Radial nodes = 4 – 2 – 1 = 1
And angular nodes,
=
= (At constant pressure)
An atomic orbital is characterized by and m.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering