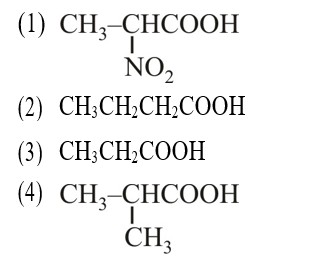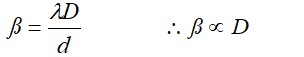The water having more dissolved O2 is:
The water having more dissolved O2 is:
Option 1 -
Water at 80°C
Option 2 -
Boiling water
Option 3 -
Polluted water
Option 4 -
Water at 4°C
-
1 Answer
-
Correct Option - 4
Detailed Solution:Solubility of gas in liquid increases on decreasing the temperature. So water at 4°C will have more dissolved O2.
Similar Questions for you
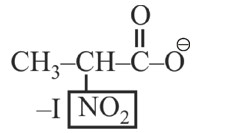
–I effect ∝ Acidic strength
+I effect ∝ Basic strength
* Most stable anion due to maximum –I effect.
* Most acidic
with increase in separation of screen from slits plane, fringe width increases.
Excessive nitrate in drinking water causes methemoglobinemia
Excessive nitrate in drinking water causes methemoglobinemia
Release of toxic/undesirable materials in the environment.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers