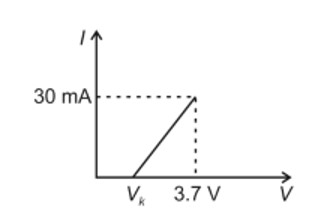
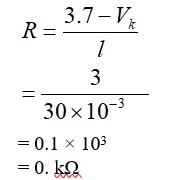To have practical applications why are cross-links required in rubber?
To have practical applications why are cross-links required in rubber?
-
1 Answer
-
This is a short answer type question as classified in NCERT Exemplar
A vulcanisation process is used to improve the physical properties of natural rubber. This process consists of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between 373 K to 415 k. Sulphur forms cross links at the reactive sites of double bonds during vulcanization, stiffening the rubber.
Similar Questions for you
Kindly go through the solution
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Monomer of neoprene polymer is
3-Chloro-1, 3-butadiene
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers