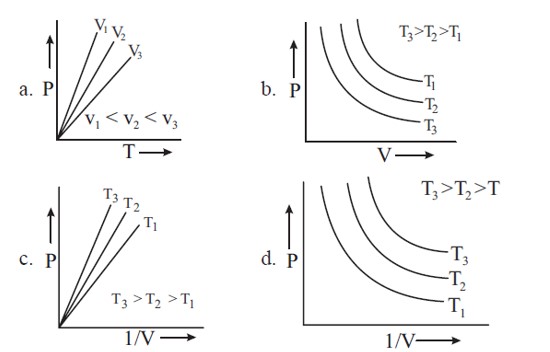
Which amongst the following options is correct graphical representation of Boyle's Law?
Which amongst the following options is correct graphical representation of Boyle's Law?
PV = nRT
P = nRT . (1/V)
Plot of P vs (1/V) would be straight line passing through origin having slope = nRT.
At high temperatures, P vs (1/V) would have greater slope.
Similar Questions for you
Correct order of van there Waals constant b for gases is CO2 > N2 > O2 > H2 > He
(A) Fluorspar – CaF2
(B) Cryolite – Na3 [AlF6]
Kindly go through the solution
Since VReal > Videal
So, Z = VReal > 1. Videal
The Answer is Maxwell? Boltzmann distribution
NH3 can be liquefied most easily due to presence of strong intermolecular H-bonding among NH3 molecules.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering