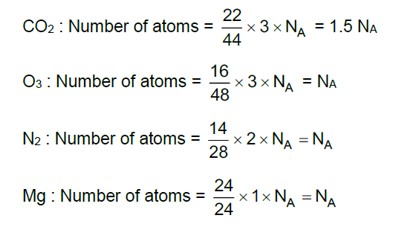Which of the following statements indicates that law of multiple proportion is being followed.
(i) Sample of carbon dioxide taken from any source will always have carbon and oxygen in the ratio 1:2.
(ii) Carbon forms two oxides namely CO2 and CO, where masses of oxygen which combine with fixed mass of carbon are in the simple ratio 2:1.
(iii) When magnesium burns in oxygen, the amount of magnesium taken for the reaction is equal to the amount of magnesium in magnesium oxide formed.
(iv) At constant temperature and pressure 200 mL of hydrogen will combine with 100 mL oxygen to produce 200 mL of water vapour.
Which of the following statements indicates that law of multiple proportion is being followed.
(i) Sample of carbon dioxide taken from any source will always have carbon and oxygen in the ratio 1:2.
(ii) Carbon forms two oxides namely CO2 and CO, where masses of oxygen which combine with fixed mass of carbon are in the simple ratio 2:1.
(iii) When magnesium burns in oxygen, the amount of magnesium taken for the reaction is equal to the amount of magnesium in magnesium oxide formed.
(iv) At constant temperature and pressure 200 mL of hydrogen will combine with 100 mL oxygen to produce 200 mL of water vapour.
This is a Multiple Choice Questions as classified in NCERT Exemplar
Option (B)
Law of multiple proportion: According to the law of multiple proportion, whe form two or more than two chemical compounds, the ratio between different elements combining with a fixed mass of the other is always in the ratio
Similar Questions for you
In the medical entrance test NEET, there can be 1 to 3 questions from this chapter. Some year, the Chemistry section of NEET has only one question from this chapter and in some other years, there can be 3 questions.
The following are the key concepts of this chapter: Compound, Elements, Rules, Law of conservation of mass, Addition and Subtraction, Atomic Mass, Law of multiple proportions, and Molecular Mass.
As the name suggests, the first chapter of the NCERT Class 11 Chemistry introduces various basic concepts of chemistry, such as the definition and importance of chemistry, atomic matter and molecular masses, the mole concept, laws of chemical combination, empirical, stoichiometry, and molecular form
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter One 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering