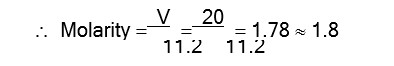With the help of suitable examples, explain the property of H2O2 that is responsible for its bleaching action?
With the help of suitable examples, explain the property of H2O2 that is responsible for its bleaching action?
This is a short answer type question as classified in NCERT Exemplar
H2O2 decomposes slowly on exposure to light.
2H2O2→ 2H2O+2O2
In daily life it is used as a hair bleach and as a mild disinfectant. As an antiseptic it is sold in the market as perhydrol.
Similar Questions for you
H2S has minimum boiling point.
PbS + 4H2O2->PbSO4 + 4H2O.
Volume strength of H2O2 = Molarity * 11.2
HF molecules are associated with strong intermolecular hydrogen bonding hence its boiling point is the highest
Compound Melting Point (K)
HF 190
HCl
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 11th Chapter Nine 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering