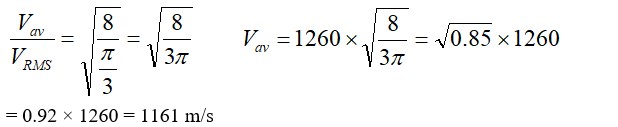13.13 A gas in equilibrium has uniform density and pressure throughout its volume. This is strictly true only if there are no external influences. A gas column under gravity, for example, does not have uniform density (and pressure). As you might expect, its density decreases with height. The precise dependence is given by the so-called law of atmospheres
n2 = n1 exp [ -mg (h2 – h1)/ kBT]
where n2, n1 refer to number density at heights h2 and h1 respectively. Use this relation to derive the equation for sedimentation equilibrium of a suspension in a liquid column:
n2 = n1 exp [ -mg NA ( - ) (h2 –h1)/ ( RT)]
where is the density of the suspended particle, and that of surrounding medium. [NA is Avogadro's number, and R the universal gas constant.] [Hint : Use Archimedes principle to find the apparent weight of the suspended particle.]
13.13 A gas in equilibrium has uniform density and pressure throughout its volume. This is strictly true only if there are no external influences. A gas column under gravity, for example, does not have uniform density (and pressure). As you might expect, its density decreases with height. The precise dependence is given by the so-called law of atmospheres
n2 = n1 exp [ -mg (h2 – h1)/ kBT]
where n2, n1 refer to number density at heights h2 and h1 respectively. Use this relation to derive the equation for sedimentation equilibrium of a suspension in a liquid column:
n2 = n1 exp [ -mg NA ( - ) (h2 –h1)/ ( RT)]
where is the density of the suspended particle, and that of surrounding medium. [NA is Avogadro's number, and R the universal gas constant.] [Hint : Use Archimedes principle to find the apparent weight of the suspended particle.]
13.13 According to law of atmospheres, we have
n2 = n1 exp [ -mg (h2 – h1)/ kBT] …..(i)
where is the number of density at height and is the number of density at height
mg is the weight of the particle suspended in the gas column
Density of the medium =
Density of the suspended particle =
Mass of one
Similar Questions for you
PV = nRT
->Pµn
->Ratio=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering