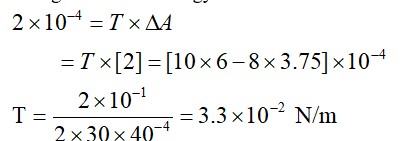2.11 Answer the following questions, which help you understand the difference between Thomson's model and Rutherford's model better.
(a) Is the average angle of deflection of particles by a thin gold foil predicted by Thomson's model much less, about the same, or much greater than that predicted by Rutherford's model?
(b) Is the probability of backward scattering (i.e., scattering of -particles at angles greater than 90°) predicted by Thomson's model much less, about the same, or much greater than that predicted by Rutherford's model?
(c) Keeping other factors fixed, it is found experimentally that for small thickness t, the number of -particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?
(d) In which model is it completely wrong to ignore multiple scattering for the calculation of average angle of scattering of -particles by a thin foil?
2.11 Answer the following questions, which help you understand the difference between Thomson's model and Rutherford's model better.
(a) Is the average angle of deflection of particles by a thin gold foil predicted by Thomson's model much less, about the same, or much greater than that predicted by Rutherford's model?
(b) Is the probability of backward scattering (i.e., scattering of -particles at angles greater than 90°) predicted by Thomson's model much less, about the same, or much greater than that predicted by Rutherford's model?
(c) Keeping other factors fixed, it is found experimentally that for small thickness t, the number of -particles scattered at moderate angles is proportional to t. What clue does this linear dependence on t provide?
(d) In which model is it completely wrong to ignore multiple scattering for the calculation of average angle of scattering of -particles by a thin foil?
12.11 About the same - The average angle of deflection -particles by a thin gold foil predicted by Thomson's model is about the same size as predicted by Rutherford's model. This is because the average angle was taken in both models.
Much less - The probability of scattering of -particles at angl
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering