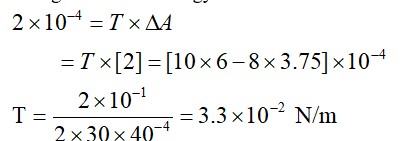A beam of monochromatic light is used to excite the electron in Li++ from the first orbit to the third orbit. The wavelength of monochromatic light found to be x * 10-10m. The value of x is_________.
[Given hc = 1242 eV nm]
A beam of monochromatic light is used to excite the electron in Li++ from the first orbit to the third orbit. The wavelength of monochromatic light found to be x * 10-10m. The value of x is_________.
[Given hc = 1242 eV nm]
We know
Energy on any orbit is given by,
[n1 = 1] (1)
For 3rd orbit
[n2 = 3]
E3 = 13.6ev
= 13.6 – (13.6 * 9)
= 114.15 * 1010m
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
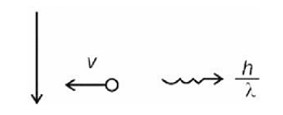
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering