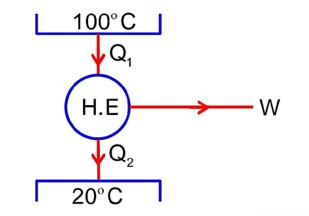
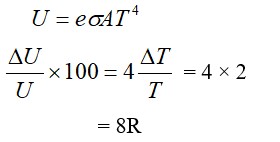A steam engine intakes 50g of steam at per minute and cools it down to If latent heat of vaporization of steam is 540 cal g1, then the heat rejected by the steam engine per minute is………….* 103 cal.
(Given : specific heat capacity of water : 1 cal g1 0C1)
A steam engine intakes 50g of steam at per minute and cools it down to If latent heat of vaporization of steam is 540 cal g1, then the heat rejected by the steam engine per minute is………….* 103 cal.
(Given : specific heat capacity of water : 1 cal g1 0C1)
4 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
Similar Questions for you
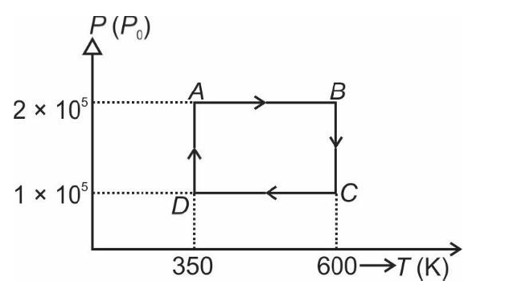
From A to B the process is isobaric
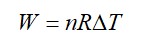
= W = 2 × R (600 - 350)
= 500 R
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering