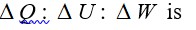Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:
Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:
Option 1 - <p>(5/2)RT<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 2 - <p>(9/2)RT</p>
Option 3 - <p>3RT</p>
Option 4 - <p>(7/2)RT</p>
6 Views|Posted 6 months ago
Asked by Shiksha User
No answers yet.
Can you answer this question?Similar Questions for you
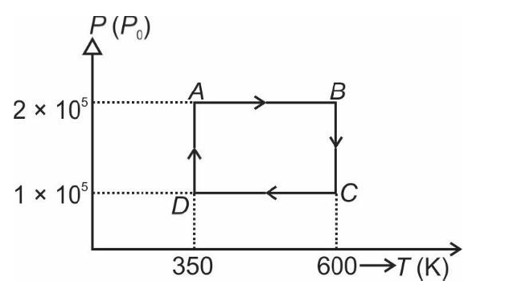
From A to B the process is isobaric
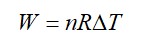
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering