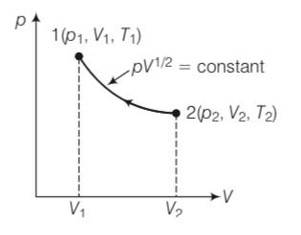
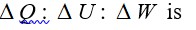Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container is shown in Fig.
(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperature T1/T2, if V2 = 2V1?
(c) Given the internal energy for one mole of gas at temperature T is (3/2) RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2 = 2V1.
Consider a P-V diagram in which the path followed by one mole of perfect gas in a cylindrical container is shown in Fig.

(a) Find the work done when the gas is taken from state 1 to state 2.
(b) What is the ratio of temperature T1/T2, if V2 = 2V1?
(c) Given the internal energy for one mole of gas at temperature T is (3/2) RT, find the heat supplied to the gas when it is taken from state 1 to 2, with V2 = 2V1.
This is a long answer type question as classified in NCERT Exemplar
pV1/2= constant
P=k/
Work done from 1 to 2
W=
from ideal equation = pV=nRT
T= pV/nR=
T=
T1= , T1=
=
U=
= RT1( )
=2p1V11/2( )
= 2p1V11/2(2 )
= 2p1V1( )= 2RT1( )
=
= RT1( )+ 2RT1( )
=
Similar Questions for you
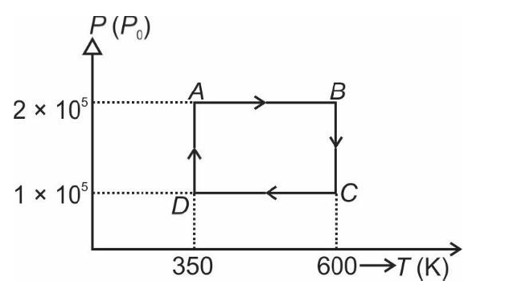
From A to B the process is isobaric
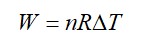
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering