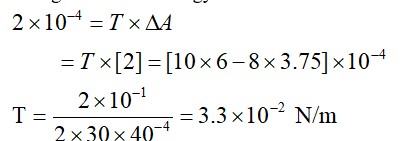Given below are two statements:
Statement I: Atoms are electrically neutral as they contain equal number of positive and negative charges.
Statement II: Atoms of each element are stable and emit their characteristic spectrum.
In the light of the above statements, choose the most appropriate answer from the options given below.
Given below are two statements:
Statement I: Atoms are electrically neutral as they contain equal number of positive and negative charges.
Statement II: Atoms of each element are stable and emit their characteristic spectrum.
In the light of the above statements, choose the most appropriate answer from the options given below.
Statement I is true as atoms are electrically neutral because they contain equal number of positive and negative charges.
Statement II is wrong as atom of most of the elements are stable and emit characteristic spectrum. But this statement is not true for every atom.
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
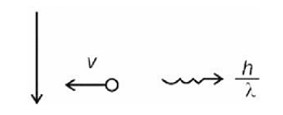
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Atoms 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering