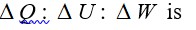Match List I with List II.
List I List II
(a) Isothermal (i) Pressure constant
(b) Isochoric (ii) Temperature constant
(c) Adiabatic (iii) Volume constant
(d) Isobaric (iv) Heat content is constant
Choose the correct answer from the options given below:
Match List I with List II.
List I List II
(a) Isothermal (i) Pressure constant
(b) Isochoric (ii) Temperature constant
(c) Adiabatic (iii) Volume constant
(d) Isobaric (iv) Heat content is constant
Choose the correct answer from the options given below:
In isothermal process, temperature is constant.
In isochoric process, volume is constant.
In adiabatic process, there is no exchange of heat.
In isobaric process, pressure is constant.
Similar Questions for you
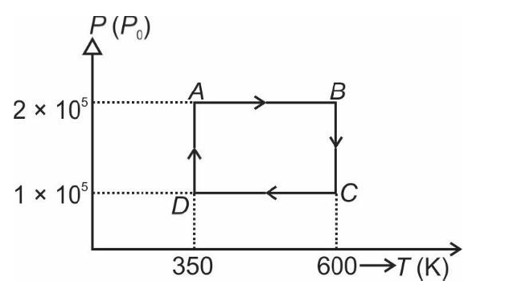
From A to B the process is isobaric
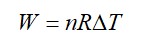
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Maths NCERT Exemplar Solutions Class 11th Chapter Eleven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering