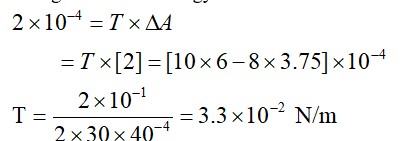The first four spectral in the Lyman series of a H-atom are λ= 1218 Å, 1028 Å, 974.3 Å and 951.4 Å. If instead of Hydrogen, we consider deuterium, calculate the shift in the wavelength of these lines.
The first four spectral in the Lyman series of a H-atom are λ= 1218 Å, 1028 Å, 974.3 Å and 951.4 Å. If instead of Hydrogen, we consider deuterium, calculate the shift in the wavelength of these lines.
This is a Long Answer Type Questions as classified in NCERT Exemplar
Explanation- as we know total energy in stationary orbit is
En=- where sign have usual meaning.
According to bohr third postulate h
-
Where is the reduced mass
Reduced mass for H=H=;me(1-me/M)
D= D; me(1-me/2M)
=me(1-me/2M)(1
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
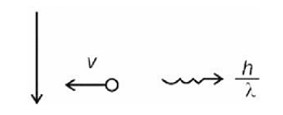
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering