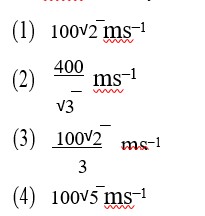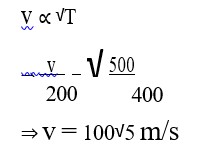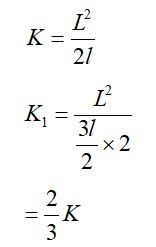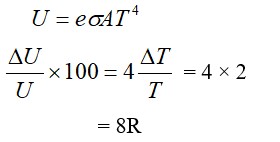The molecules of a given mass of a gas have r.m.s. velocity of 200 m/s at 127°C and 1.0 * 105 N/m2 pressure. When the temperature and pressure of the gas are respectively, 227°C and 0.05 * 105 N/m2, the r.m.s. velocity of its molecules is :
The molecules of a given mass of a gas have r.m.s. velocity of 200 m/s at 127°C and 1.0 * 105 N/m2 pressure. When the temperature and pressure of the gas are respectively, 227°C and 0.05 * 105 N/m2, the r.m.s. velocity of its molecules is :
Option 1 - <p>A</p>
Option 2 - <p>B</p>
Option 3 - <p>C</p>
Option 4 - <p>D</p>
1 Views|Posted 3 months ago
Asked by Shiksha User
1 Answer
A
Answered by
3 months ago
Correct Option - 4
Detailed Solution:
Similar Questions for you
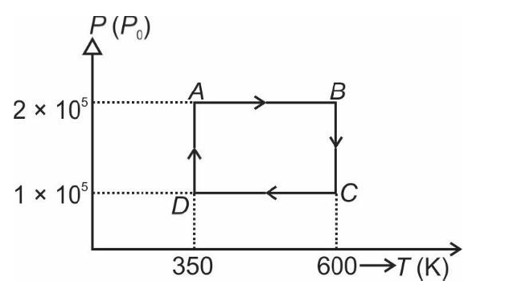
From A to B the process is isobaric
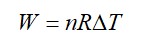
= W = 2 × R (600 - 350)
= 500 R
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering