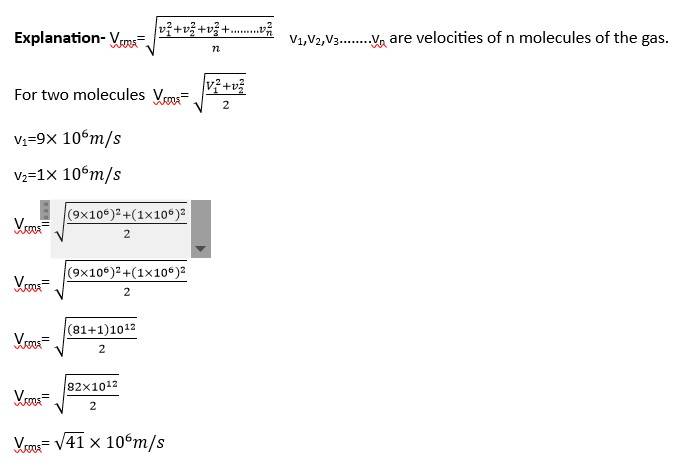
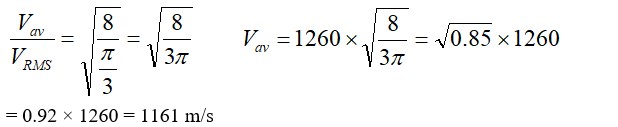Two molecules of a gas have speeds of 9 x 106 m s-1 and 1 x 106 ms−1 , respectively. What is the root mean square speed of these molecules.
Two molecules of a gas have speeds of 9 x 106 m s-1 and 1 x 106 ms−1 , respectively. What is the root mean square speed of these molecules.
2 Views|Posted 8 months ago
Asked by Shiksha User
1 Answer
P
Answered by
8 months ago
This is a short answer type question as classified in NCERT Exemplar
Similar Questions for you
PV = nRT
->Pµn
->Ratio=
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

physics ncert solutions class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering