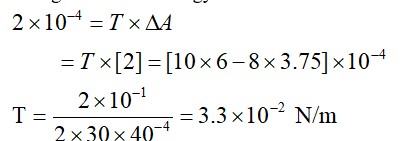What was Thomson's Model of the Atom or Plum Pudding in class 12 Atoms?
What was Thomson's Model of the Atom or Plum Pudding in class 12 Atoms?
As per the NCERT Textbooks, Thomson proposed a Atomic Structure of Atom that tells" An atom consists of a positively charged sphere in which the electrons are embedded like the seeds are embedded in watermelon. This model is often compared to a pudding or watermelon with electrons distributed like r
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
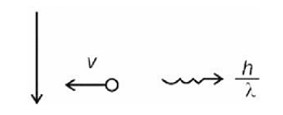
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Physics Ncert Solutions Class 12th 2026
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering