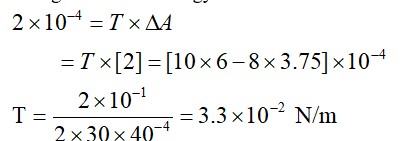Which of the following is true?
Which of the following is true?
Option 1 - <p>The Lyman series has a continuous spectrum</p>
Option 2 - <p>The Balmer series is a line spectrum in the UV region</p>
Option 3 - <p>The Paschan series is a line spectrum in the infrared region</p>
Option 4 - <p>Spectral series formula is derived from Rutherford’s model of the hydrogen atom</p>
1 Views|Posted 4 months ago
Asked by Shiksha User
1 Answer
A
Answered by
4 months ago
Correct Option - 3
Detailed Solution:
The spectrum of H-atom is formulated and drawn by Bohr's model. These spectrums are line spectrum. Lyman series lies in UV region, Balmer Partly lies UV and partly in the visible region and Paschen series lies in the infrared region.
Similar Questions for you
Kindly go through the solution
Change in surface energy = work done
|DE0| = –10.2
]
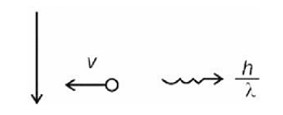
= 3 m/s
n = 4
Number of transitions =
Kinetic energy: Potential energy = 1 : –2
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Physics Atoms 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering