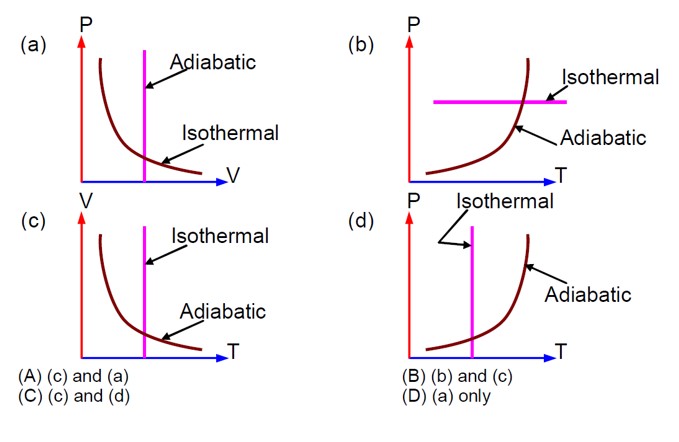
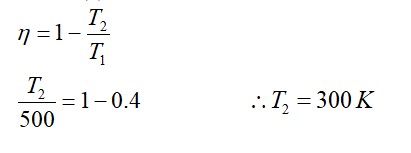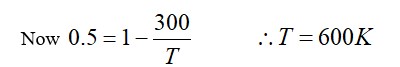Which one is the correct option for the two different thermodynamic processes?
Which one is the correct option for the two different thermodynamic processes?
Option 1 - <p>(c) and (a)<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
Option 2 - <p>(b) and (c)</p>
Option 3 - <p>(c) and (d)</p>
Option 4 - <p>(a) only</p>
28 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
A
Answered by
5 months ago
Correct Option - 1
Detailed Solution:
Pressure decreases with an increase in volume in both isothermal and adiabatic processes. In an adiabatic process, as volume decreases, pressure increases with the increase in temperature.
Similar Questions for you
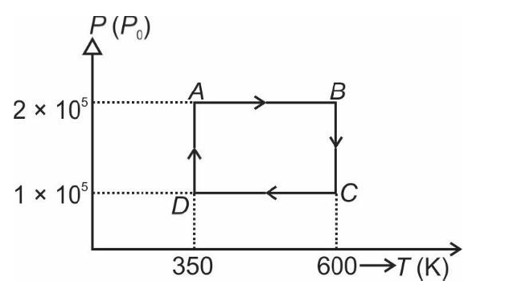
From A to B the process is isobaric
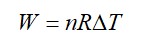
= W = 2 × R (600 - 350)
= 500 R
Heat is path dependent so path function but internal energy does not depend on path chosen.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering