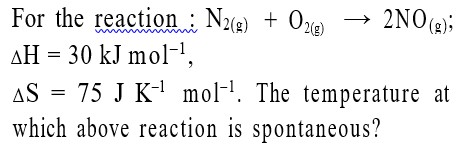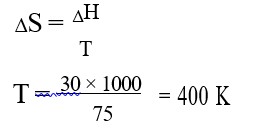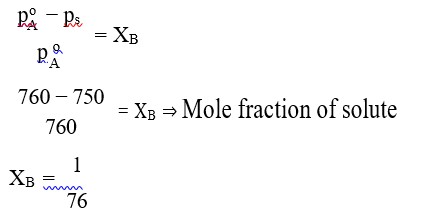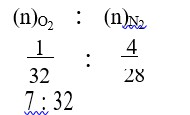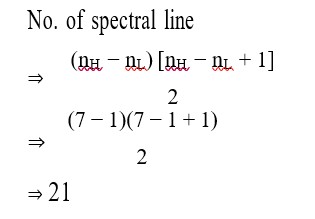Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoNew Question
4 months agoNew Question
4 months agoContributor-Level 10
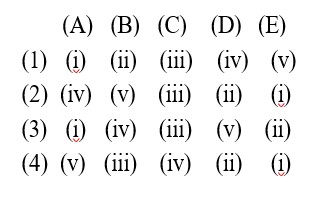
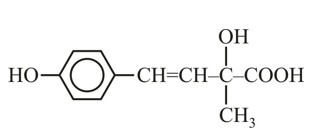
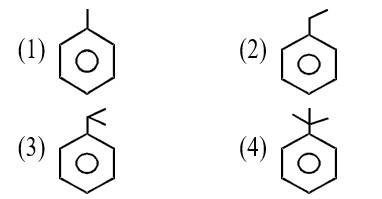
It has chiral centre and differently di substituted double bonded carbon atoms.
New Question
4 months agoContributor-Level 10
Cr3+ion is a most stable in aqueous solution due to. t2g half filled configuration
New Question
4 months agoContributor-Level 10
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
New Question
4 months agoContributor-Level 10
They both offer the best education to international students. If we compare them in terms of MS in CS, then the cost of study at Georgia Tech is lower than at UCL. Students can check the below table for more detail:
| University | First Year Tuition Fee | Ranking |
|---|---|---|
| Georgia Institute of Technology | INR 17 L | #97 |
| University College London | INR 37 L | #9 |
New Question
4 months agoContributor-Level 10
pH = 13, pOH = 1
[OH–] = 10–1
no. of moles OH– in 1L = 10–1
No. of moles of OH– in 1 ml = 10–4 No. of [OH–] ions = 6.02 × 1023 × 10–4
⇒ 6.02 × 1019
New Question
4 months agoContributor-Level 9
BPSC Prelims Result is released. To download the BPSC result, Go bpsc.bihar.gov.in. Click on BPSC 71st Prelims Result in the “What's New” section, and the PDF will appear. You can download and save it for later use.
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts