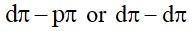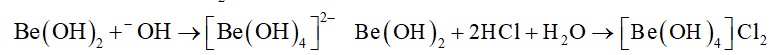Ask & Answer: India's Largest Education Community
All Questions
New Question
4 months agoContributor-Level 10
Group?17, elements have very high ionisation enthalpy due to increase in atomic size, ionization enthalpy decreases down the group.
New Question
4 months agoContributor-Level 10
Sucrose is non-reducing sugar. It does not reduce Fehling solution.
New Question
4 months agoContributor-Level 10
In the metallurgy of aluminium, purified Al2O3 is mixed with Na3AIF6 or CaF2 which lowers the melting point of the mixture and brings conductivity.
New Question
4 months agoContributor-Level 10
Heavier element of p block do not from pπ− pπ bonds as their atomic orbital are so large and diffius that they cannot have effecitve overlapping.
New Question
4 months agoContributor-Level 10
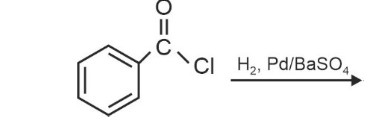
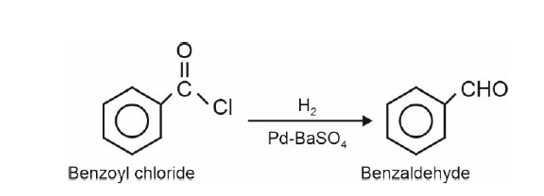
Acyl chloride is hydrogenated over catalyst, palladium or barium sulphate. This reaction is called Rosenmund reaction.
New Question
4 months agoContributor-Level 10
The inertness of
New Question
4 months agoContributor-Level 10
Sodium carbonate is a white crystalline solid which exists as a decahydrate, 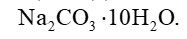
New Question
4 months agoNew Question
4 months agoContributor-Level 10
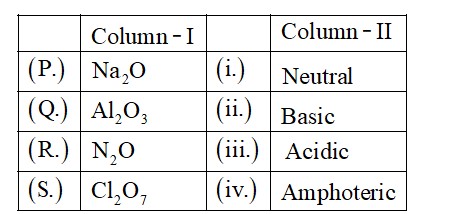
Bond angles give some idea regarding the distribution of orbitals around the central atom in a molecule/complex ion and hence it helps us in determining its shape
New Question
4 months agoContributor-Level 10
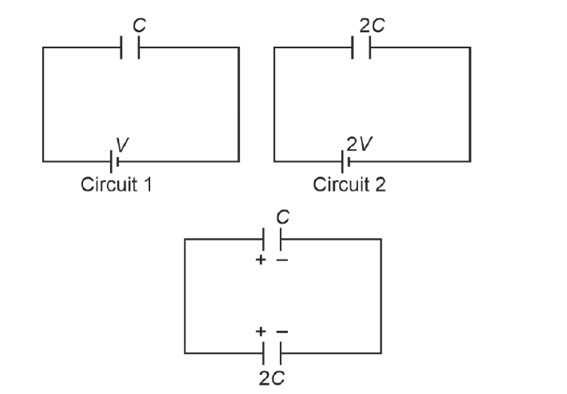
Charge on C1 = CV
Charge on C2 = 4CV
When connected in parallel
x = 50
New Question
4 months agoContributor-Level 10
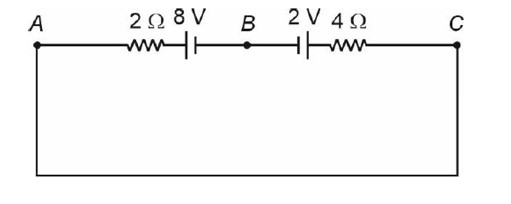
Current in circuit (I) = = 1 A.
So, VC – 4 (1) – 2 + 8 – 2 (1) = VA
VC – 6 – 2 + 8 = VA
VC – VA = 0 V
New Question
4 months agoContributor-Level 10
Dv = a (t + 1 – t)
a = 50 m/s2
u = 120 – 25 (2t +1)
In (t + 2)th second
= u + 25 (2t + 3)
= 120 – 25 (2t) – 25 + 25 (2t) + 75
S' = 170 m
New Question
4 months agoContributor-Level 10
RHUL, situated in Egham, Surrey, is a public university based in UK. The university provides an inclusive environment to the international crowd and a high placement rate, pointing to bright future prospect. The campus area is known to have greenery and various notable landmarks.
New Question
4 months agoContributor-Level 10
The University of Wolverhampton is a public university, therefore, tuition fees for international students and domestic students may vary. Also, the tuition fees varies from course to course and depends on the type of course and level of study. The average tuition fee for some popular courses at the University of Wolverhampton are givne below:
Courses Average Fees
BE/BTech INR 13 L
MS INR 15 L
BBA INR 13 L
MIM INR 14.2 L - 15.1 L
Register to get relevant
Questions & Discussions on your feed
Ask & Answer
Panel of Experts