What are the key assumptions of the kinetic theory of an ideal gas?
The kinetic theory is based on many key assumptions, such as the following:
- The individual gas molecules volume is almost non-existing when compared to the total volume of the gas.
- A gas consists of many small, identical, hard spherical particles.
- The collisions between the gas particles and the contai
Similar Questions for you
Yes, there are many numerical problems in class 11 Physics. All important formulas must be on figure tips.
Class 11 Physics Chapter 11 is Thermodynamics. It is one of the most important topic in Physics.
There are three main processes Isothermal, adiabatic and cyclic process. In isothermal, the system is thermally conductive and the temperature remains constant. In adiabatic process, the system is thermally isolated and there is no change in heat temperature. The system returns to its initial stage
4.22
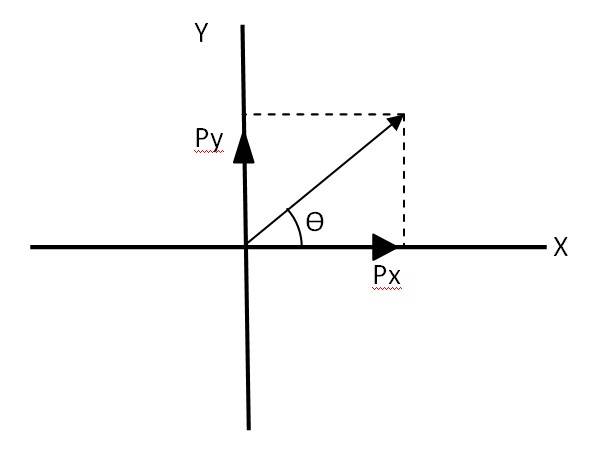
Let us consider a vector . The equation can be written as
Px = Py = 1 = = = …….(i)
So the magnitude of vector + =
Let be the angle made by vector , with the x axis as given in the above fi
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering

