What is the significance of the Maxwell-Boltzmann distribution law according to the kinetic theory of gases class 11?
According to the kinetic theory of gases class 11, the Maxwell-Boltzmann distribution law describes the gas molecules' distribution of speeds (or velocities). All molecules of gas do not move at the same speed, some move fast, some are slow and most move at an average speed. This law provides a math
Similar Questions for you
Yes, there are many numerical problems in class 11 Physics. All important formulas must be on figure tips.
Class 11 Physics Chapter 11 is Thermodynamics. It is one of the most important topic in Physics.
There are three main processes Isothermal, adiabatic and cyclic process. In isothermal, the system is thermally conductive and the temperature remains constant. In adiabatic process, the system is thermally isolated and there is no change in heat temperature. The system returns to its initial stage
4.22
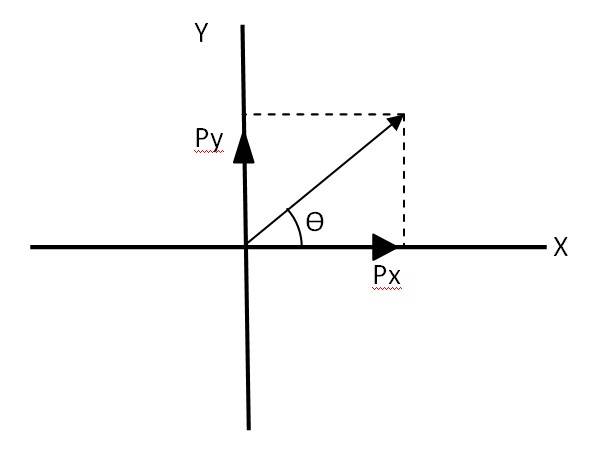
Let us consider a vector . The equation can be written as
Px = Py = 1 = = = …….(i)
So the magnitude of vector + =
Let be the angle made by vector , with the x axis as given in the above fi
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering

