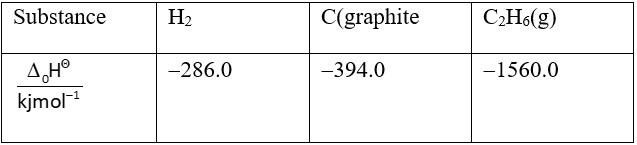
At and 1 atm pressure, the enthalpies of combustion are as given below:
The enthalpy of formation of ethane is
At and 1 atm pressure, the enthalpies of combustion are as given below:
The enthalpy of formation of ethane is
Option 1 - <p>+54.0 kJ mol<sup>-1</sup> </p>
Option 2 - <p>-68.0 kJ mol<sup>-1</sup></p>
Option 3 - <p>-86.0 kJ mol<sup>-1</sup></p>
Option 4 - <p>+97.0 kJ mol<sup>-1</sup></p>
2 Views|Posted 5 months ago
Asked by Shiksha User
1 Answer
R
Answered by
5 months ago
Correct Option - 3
Detailed Solution:
= (-788 – 858 + 1560) KJ/mole = -86 kJ/mole
Similar Questions for you
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering