An organic compound P contains 62.07% carbon and 10.34% hydrogen and rest oxygen. Its vapour density is 29. This compound does not react with sodium meta, but its 2.9g combine with Xg of bromine (to give dibromo addition product). Find out value of (Y-X). (Where Y is total number of possible isomers of given organic compound P)
[Atomic mass : Br = 80]
An organic compound P contains 62.07% carbon and 10.34% hydrogen and rest oxygen. Its vapour density is 29. This compound does not react with sodium meta, but its 2.9g combine with Xg of bromine (to give dibromo addition product). Find out value of (Y-X). (Where Y is total number of possible isomers of given organic compound P)
[Atomic mass : Br = 80]
Molecular formula from calculation comes to be C3H6O i.e., it stands for the following compounds.
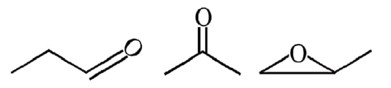
No addition reaction with Br2
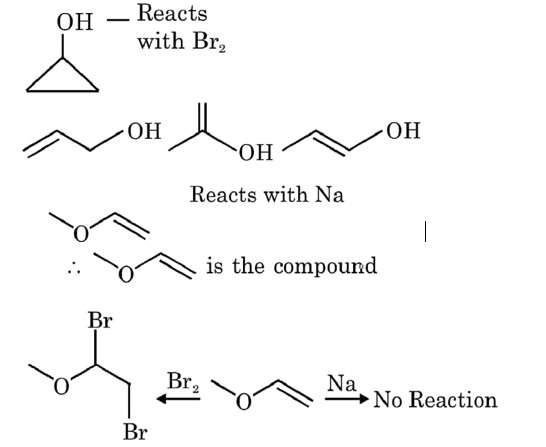
VD * 2 = MM = 29 * 2 = 58
2.9 g of ether will combines with
Y = 9 and X = 8
Y - X = 9 - 8 = 1
Similar Questions for you
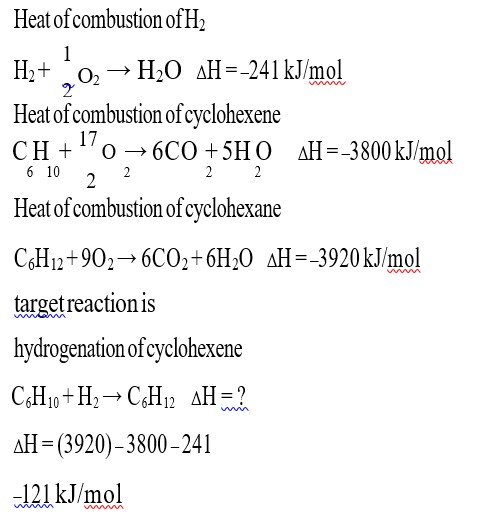
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Organic Chemistry 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering