At 25°C, 50 g of iron reacts with HCl to form FeCl?. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is __________ J. (Round off to the nearest integer).
[Given: R= 8.314 J mol?¹ K?¹. Assume hydrogen is an ideal gas]
[Atomic mass of Fe is 55.85u]
At 25°C, 50 g of iron reacts with HCl to form FeCl?. The evolved hydrogen gas expands against a constant pressure of 1 bar. The work done by the gas during this expansion is __________ J. (Round off to the nearest integer).
[Given: R= 8.314 J mol?¹ K?¹. Assume hydrogen is an ideal gas]
[Atomic mass of Fe is 55.85u]
The reaction is Fe (s) + 2HCl (aq) → FeCl? (aq) + H? (g). 50 g of Fe corresponds to 0.89 moles, which produces 0.89 moles of H? (g). The work done by the gas is calculated using W = -Δn_gasRT. The work done by the gas is the positive value, 2218 J.
Answer: 2218 J
Similar Questions for you
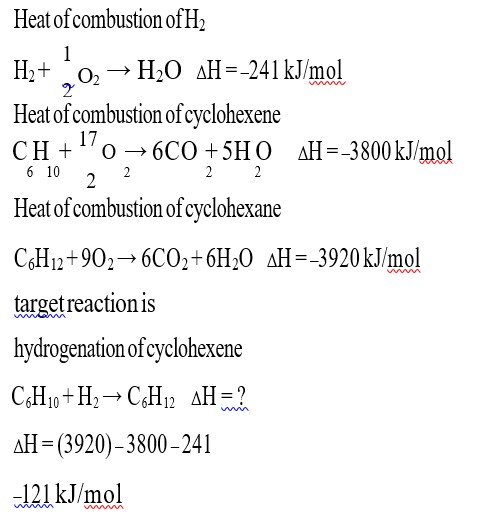
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Ncert Solutions Class 11th 2023
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering