If the work function of a metal is 6.63 * 10-19J, the maximum wavelength of the photon required to remove a photoelectron from the metal is _______nm. (Nearest integer)
[Given h = 6.63 * 10-34 Js, and c = 3 * 108 ms-1]
If the work function of a metal is 6.63 * 10-19J, the maximum wavelength of the photon required to remove a photoelectron from the metal is _______nm. (Nearest integer)
[Given h = 6.63 * 10-34 Js, and c = 3 * 108 ms-1]
Work function,
Threshold wavelength
Using ;
=300 * 10-9 m = 300 nm
Similar Questions for you
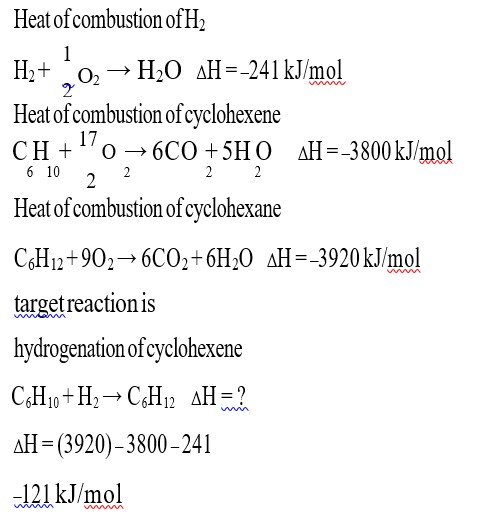
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry NCERT Exemplar Solutions Class 12th Chapter Seven 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering