Complete combustion of 750g of an organic compound provides 420 g of CO2 and 210 g of H2O.
The percentage composition of carbon and hydrogen in organic compound is 15.3 and _________ respectively. (Round off to the nearest Integer).
Complete combustion of 750g of an organic compound provides 420 g of CO2 and 210 g of H2O.
The percentage composition of carbon and hydrogen in organic compound is 15.3 and _________ respectively. (Round off to the nearest Integer).
Moles of carbon in organic compound = Moles of carbon in CO?
n_c = 420 / 44 moles
Mass of carbon in organic compound = (420 / 44) * 12 = 114.54 g
Moles of hydrogen in compound = 2 * moles of H? O
n_H = 2 * (210 / 18) moles
Mass of hydrogen = 2 * (210 / 18) g = 23.33 g
% of H = (23.33 / 750) * 100 = 3.11%
Similar Questions for you
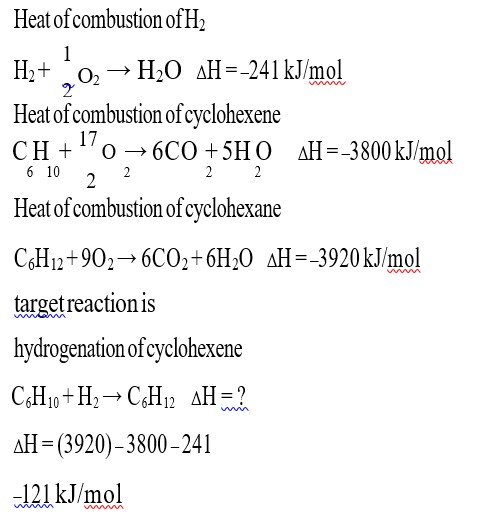
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering