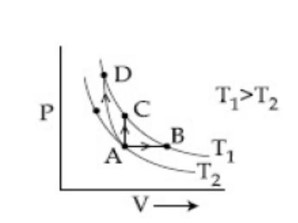
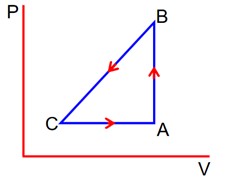
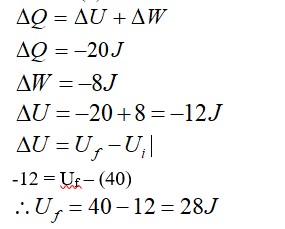Three different processes that can occur in an ideal monoatomic gas are shown in the P vs V diagram. The paths are labeled as A → B, A → C and A → D. The change in internal energies during these process are taken as EAB, EAC and EAD and the work done by WAB, WAC and WAD. The correct relation between these parameters are:
Three different processes that can occur in an ideal monoatomic gas are shown in the P vs V diagram. The paths are labeled as A → B, A → C and A → D. The change in internal energies during these process are taken as EAB, EAC and EAD and the work done by WAB, WAC and WAD. The correct relation between these parameters are:
Option 1 - <p>EAB < EAC < EAD, WAB > 0, WAC > WAD</p>
Option 2 - <p>EAB = EAC = EAD, WAB > 0, WAC = 0, WAD > 0</p>
Option 3 - <p>EAB > EAC > EAD, WAB < WAC < WAD</p>
Option 4 - <p>EAB = EAC < EAD, WAB > 0, WAC = 0, WAD < 0<br><!-- [if !supportLineBreakNewLine]--><br><!--[endif]--></p>
3 Views|Posted 5 months ago
Asked by Shiksha User
No answers yet.
Can you answer this question?Similar Questions for you
From first law of thermodynamics
From first law of thermodynamics
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering