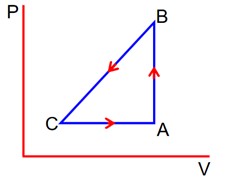
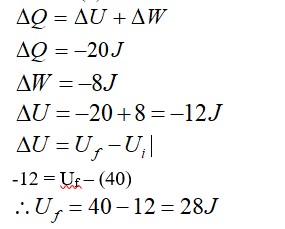A sample of an ideal gas is taken through the cyclic process ABCA as shown in figure. It absorbs, 40 J of heat during the part AB, no heat during BC and rejects 60 J of heat during CA. A work of 50 J is done on the during the part BC. The internal energy of the gas at A is 1560 J. The work done by the gas during the part CA is:
A sample of an ideal gas is taken through the cyclic process ABCA as shown in figure. It absorbs, 40 J of heat during the part AB, no heat during BC and rejects 60 J of heat during CA. A work of 50 J is done on the during the part BC. The internal energy of the gas at A is 1560 J. The work done by the gas during the part CA is:
Given
QAB = +40J
QBC = 0J
QCA = -60J
WCA =?
WBC = -50J (on the gas)
UA = 1560J
1st Law on path A to B
WAB +
Þ 0 +
Þ UB = UA + 40
= 1560 + 40
UB = 1600 J
1st Low on path B to C
QBC =
Þ 0 = Uc – UD – 50
UC = UB + 50
= 1600 +
Similar Questions for you
From first law of thermodynamics
From first law of thermodynamics
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering