10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
-
1 Answer
-
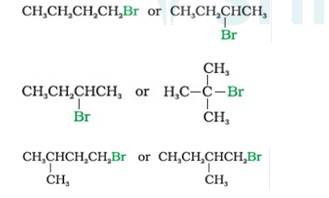
In SN2 Reaction, the product will be formed in a single step with no intermediates. Nucleophile attack will be easier for simple halides than hindered haloalkanes. Therefore,

- Bromobutane (1°)reacts faster than 2-Bromobutane (2°)
- 2-Bromobutane (2°) reacts faster than 2-Bromo-2-methylpropane or tert-Butyl bromide (3°) 1-Bromo-3-methyl butane reacts faster than the 1-Bromo-2-methyl bu
- tane as the former is less hindered with respect to leaving halide than the later which is more hindered comparatively
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






