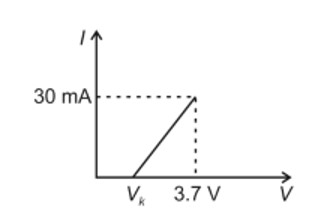
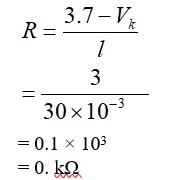0.01 M KMnO4 solution was added to 20.0mL of 0.05 M Mohr’s salt through a burette. The initial reading of 50mL burette is zero. The volume of KMnO4 solution left in the burette after the end point is__________ mL. (nearest integer)
0.01 M KMnO4 solution was added to 20.0mL of 0.05 M Mohr’s salt through a burette. The initial reading of 50mL burette is zero. The volume of KMnO4 solution left in the burette after the end point is__________ mL. (nearest integer)
-
1 Answer
-
n-factor of KMnO4 in acidic medium = 5
n-factor of Mohr's salt = 1
meq of KMnO4 = meq of Mohr's salt
0.01 * 5 * V = 0.05 * 1 * 20
Volume of KMnO4 used, V = 20 mL
So; Volume of KMnO4 left in burette = 50 -20mL
= 30 mL
Similar Questions for you
Kindly go through the solution
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Monomer of neoprene polymer is
3-Chloro-1, 3-butadiene
CH2 = CHCN (acrylonitrile) is a monomer of orlon
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers