0.25g of an organic compound containing chlorine gave 0.40g of silver chloride in Carius estimation. The percentage of chlorine present in the compound is___________. [ in nearest integer]
(Given: Molar mass of Ag is 108 g mol-1 and that of Cl is 35.5 g mol-1)
0.25g of an organic compound containing chlorine gave 0.40g of silver chloride in Carius estimation. The percentage of chlorine present in the compound is___________. [ in nearest integer]
(Given: Molar mass of Ag is 108 g mol-1 and that of Cl is 35.5 g mol-1)
-
1 Answer
-
Moles of chlorine in the given compound = Moles of chlorine in AgCl
= moles of AgCl
Mass of chlorine =
= 0.098 g
Similar Questions for you
4-ethoxycarbonylpent-3-enoic acid
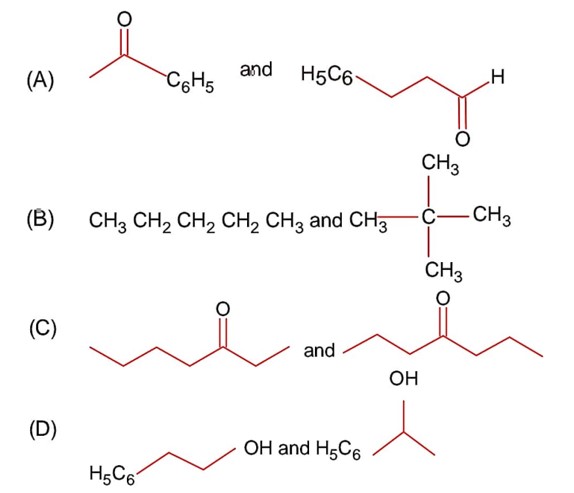
In metamers, distribution of alkyl groups are changed with respect to polyvalent functional groups.
Sodium fusion extract is boiled with conc. HNO3 to remove NaCN and Na2S.
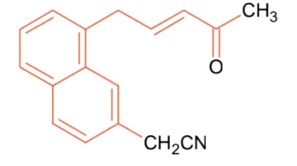
Number of sp2 hybridized carbon in A = 2
Kindly consider the following figure
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers