0.4g mixture of NaOH, Na2CO3 and some inert impurities was first titrated with using phenolphthalein as an indicator, 17.5mL of HCl was required at the end point . After this methyl orange was added and titrated. 1.5mL of same HCl was required for the next end point. The weight percentage of Na2CO3 in the mixture is----------. (Rounded – off to the nearest integer)
0.4g mixture of NaOH, Na2CO3 and some inert impurities was first titrated with using phenolphthalein as an indicator, 17.5mL of HCl was required at the end point . After this methyl orange was added and titrated. 1.5mL of same HCl was required for the next end point. The weight percentage of Na2CO3 in the mixture is----------. (Rounded – off to the nearest integer)
NaOH + Na2CO3
(1) When Hph is added
m.e NaOH + (m.e = milli equivalents)
(2) When MeOH is added after Hph
Similar Questions for you
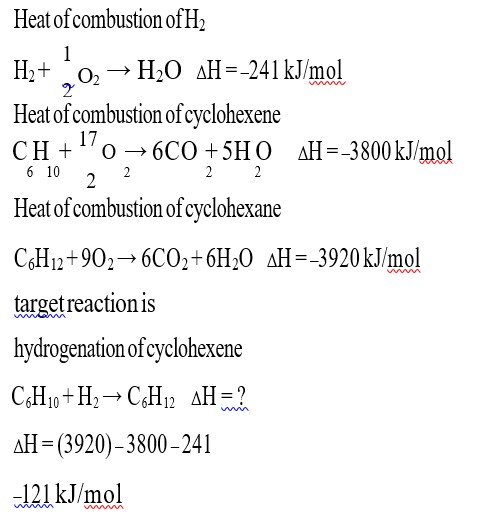
CH3COOH + NaOH → CH3COONa + H2O
ΔH = –50.6 kJ/mol
NaOH + SA [HCl] → NaCl + H2O
ΔH = –55.9 kJ/mol
the value of ΔH for ionisation of CH3COOH
⇒ ΔH = +55.9 – 50.6
5.3 kJ/mol
Kindly consider the solution
Fact.
Kindly go through the solution
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
Learn more about...

Chemistry Some Basic Concepts of Chemistry 2025
View Exam DetailsMost viewed information
SummaryDidn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
See what others like you are asking & answering