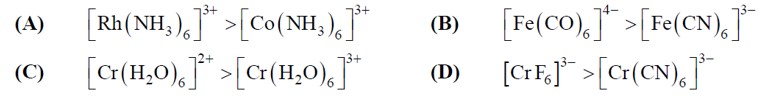1 mol of an octahedral metal complex with formula MCl3
2L on reaction with excess of AgNO3 gives 1 mol of AgCl. The denticity of Ligand L is________(integer answer)
1 mol of an octahedral metal complex with formula MCl3 2L on reaction with excess of AgNO3 gives 1 mol of AgCl. The denticity of Ligand L is________(integer answer)
-
1 Answer
-
MCl3.2L => octahedral complex
It means one Cl- ion present in ionization sphere.
For octahedral complex co-ordination numbers is 6
is bidentate ligand i.e. its denticity is 2.
Similar Questions for you
CoCl3.NH3 + AgNO3
x = 5
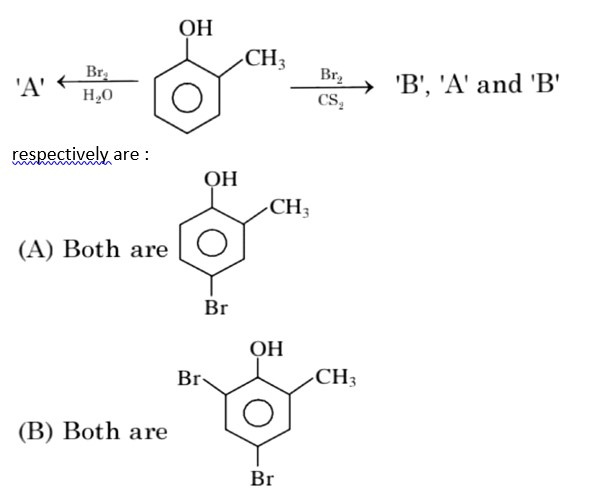
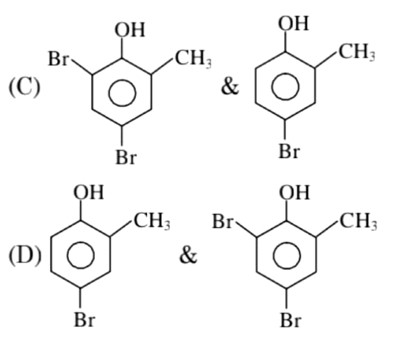
In H2O (polar solvent) dibromophenol derivative and in CS2 (non-polar solvent moneobromo phenol derivate is obtained.
3d => 4d => 5d CFSE increases for the same ligands.
Factual
⇒ leaching methods is used for those metal in which metal is more soluble than impurities and these are Al, Au, Ag, low grade Cu
σ bonded organometallic compound ⇒ M – C
σ-bond
and in π – bonded organo metallic compound
M – C
π bond
In ferrocene, there is π-bond
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers