10.13 Which one of the following has the highest dipole moment?
(i) CH2Cl 2 (ii) CHCl 3 (iii) CCl4
10.13 Which one of the following has the highest dipole moment?
(i) CH2Cl 2 (ii) CHCl 3 (iii) CCl4
-
1 Answer
-
Dipole moment of a molecule depends on the electronegativity difference between atoms bonded covalently and geometry of the molecule (how far the atoms are from each other). Dipole moment is important to understand the polarity of a molecule.
The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:-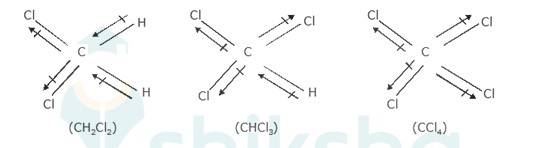
CCl4 being symmetrical has zero dipole moment. In CHCl3, the resultant of the two C-Cl dipole moments is opposed by the resultant of C-H and C-Cl bonds. Since the dipole Moment of latter resultant is expected to be smaller than the former, CHCl3 has a finite dipo
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






