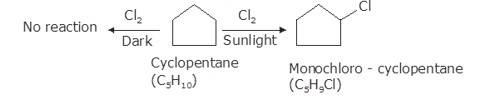
10.14 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
10.14 A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
-
1 Answer
-
As per the molecular formula , one thing can be confirmed; the hydrogen is either cycloalkane or it is an alkene. However, alkenes readily react with chlorine in even dark because of the double bond. Therefore, it is possible that the hydrocarbon is cycloalkane.
Now, let us consider the reactivity. Cycloalkanes are the saturated hydrocarbons with no double bonds. These do not react with Chlore in dark because such a reaction needs UV light for initiating the process of free radical substitution. Haloalkane and Haloarenes NCERT solutions cover this as well as other questions in further detail.
In bright sunlight, the UV light star
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers