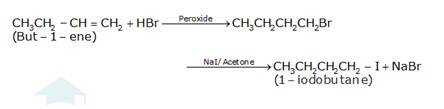
10.16 Write the equations for the preparation of 1-iodobutane from (i) 1-Butanol (ii) 1-Chlorobutane (iii) But-1-ene.
10.16 Write the equations for the preparation of 1-iodobutane from (i) 1-Butanol (ii) 1-Chlorobutane (iii) But-1-ene.
-
1 Answer
-
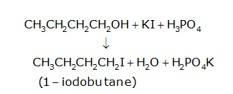
(a) 1-butanol is treated with KI in the presence of H3PO4 where the OH is being replaced by the iodine and gives H2O and KH2PO4 as the by-product with 1-iodobutane as the final
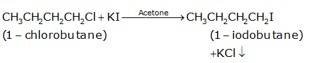
(b) The conversion of 1-chlorobutane to 1-iodobutane simply takes place by treating the reactant with KI in the presence of acetone. The iodine from KI normally replaces the chlorine from the reactant and gives 1-iodobutane as the final product with KCl as the by product.
(c) The conversion of but-1-ene to 1-iodobutane takes place according to anti-markovnikoff (positive charged ion H+ goes to the carbon which has less number of hydrogens and negative part Br-
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers