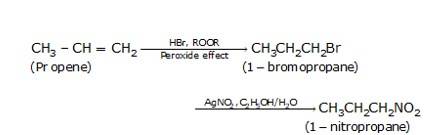
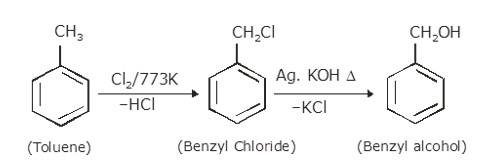
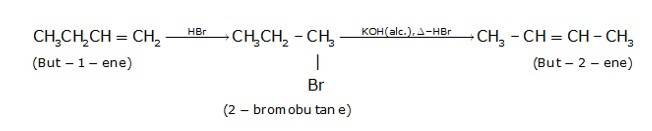
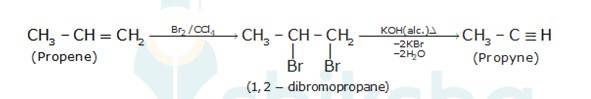10.21 How will you bring about the following conversions? (i) Ethanol to but-1-yne (ii) Ethane to bromoethene (iii) Propene to 1-nitropropane (iv) Toluene to benzyl alcohol (v) Propene to propyne (vi) Ethanol to ethyl fluoride (vii) Bromomethane to propanone (viii) But-1-ene to but-2-ene (ix) 1- Chlorobutane to n-octane (x) Benzene to biphenyl.(Advanced)
10.21 How will you bring about the following conversions? (i) Ethanol to but-1-yne (ii) Ethane to bromoethene (iii) Propene to 1-nitropropane (iv) Toluene to benzyl alcohol (v) Propene to propyne (vi) Ethanol to ethyl fluoride (vii) Bromomethane to propanone (viii) But-1-ene to but-2-ene (ix) 1- Chlorobutane to n-octane (x) Benzene to biphenyl.(Advanced)
-
1 Answer
-
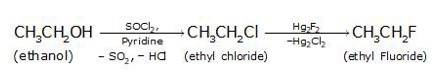
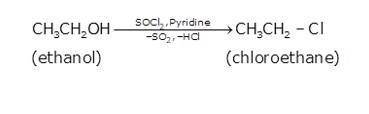
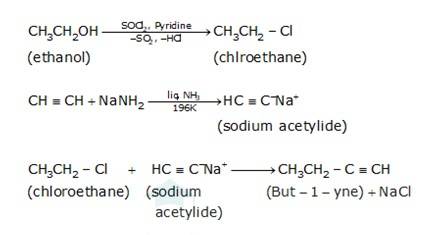
(i) Since the conversion of ethanol to but-1-yne involves the addition the two extra carbons that is why the conversion is carried out in 3 steps.
- In the first step ethanol is treated with thionyl chloride (SOCl2) in the presence of pyridine to give chloroethane
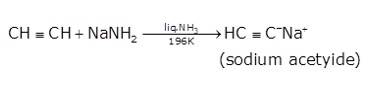
- In the second step the acetylene is treated with sodamide(NaNH2) in the presence of liquid ammonia to give sodium acetylide
- The last step involves the reaction between chloroethane and sodium acetylide to give the final product as the But-1-yne and NaCl as the by-product.
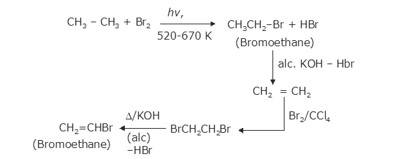
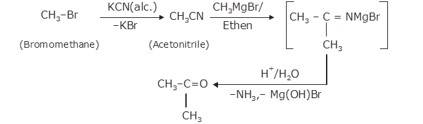
- The bromination of ethane (Br2 in the presence of light at 520-670K) gives bromoethane which on further treatme
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers