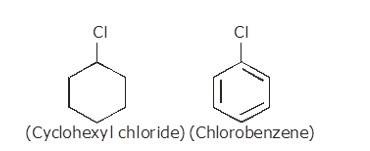
10.22 Explain why (i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) Alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous conditions?
10.22 Explain why (i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) Alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous conditions?
-
1 Answer
-
Let us explain each question from Haloalkane and Haloarenes chapter one by one. Students who are currently in school and plan to take the CBSE board exam for class 12th soon, need to prepare all these questions. Let us get started.
(i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
Sp2 hybrid carbon (s-character=33.33%) in chlorobenzene is more electronegative than a sp3-hybrid carbon (s-character=25%) in cyclohexylchloride, due to greater s-character. Thus, Carbon atom of chlorobenzene has less tendency to release electrons to Cl than carbon atom of cyclohexylchloride.
Chlorine atom in chlorobenzene is atta
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers