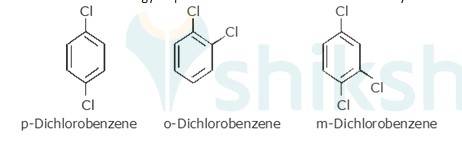
10.28 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
10.28 p-Dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
-
1 Answer
-
p-Dichlorobenzene is more symmetrical than o-and m-isomers. Because it fits more closely and easily in the crystal lattice than o-and m-isomers. Therefore, more energy is required to break the crystal lattice of p-dichlorobenzene. Therefore, p-dichlorobenzene is symmetrical and has a higher melting point and lower solubility than o-and m-isomers due to strong force of attraction in crystal.
Therefore more energy required to break lattice and will not easily be soluble.
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






