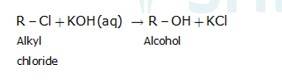10.30 The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
10.30 The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
-
1 Answer
-
An aqueous solution, KOH almost completely ionizes to give OH-ions. OH- ion is a strong nucleophile (see the imp. note below), which leads the alkyl chloride to undergo a nucleophilic substitution reaction to form alcohol.
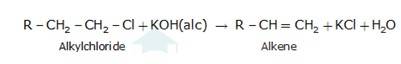
On the other hand, an alcoholic solution of KOH contains alkoxide (RO-) ion, it is a strong base. Thus, it can abstract a hydrogen ion from the beta-carbon of the alkyl chloride and form an alkene by eliminating a molecule of HCl.OH- ion is a much weaker base than RO- ion. Also, OH- ion is highly solvated (because more energy is released on solvation)in an aqueous solution and as a result, the basic character of OH-io
...more
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers