10.8 In the following pairs of halogen compounds, which compound undergoes faster SN 1 reaction?
10.8 In the following pairs of halogen compounds, which compound undergoes faster SN 1 reaction?
-
1 Answer
-
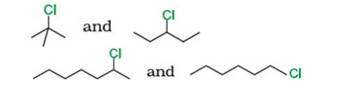

Reason: SN1 occurs in two steps. In the first step, carbocation forms. The rate of reaction in SN1 depends upon the stability of the carbocation, greater the stability faster the reaction. Tertiary (3°) carbocation is more stable than secondary (2°), which is further stable than primary (1°)
By using this
- Tert-Butyl Chloride (3°) reacts faster than 3-Chloropentane (2°)
- 2-Chloro heptane (2°) is more reactive than 1-Chloro hexane (1°)
Similar Questions for you
Photodiode in reverse bias mode is used as intensity measuring device.
Tertiary haloalkane does not undergo SN2 reaction
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers






