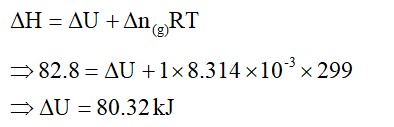10. Calculate the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at -10.0°C. A, ΔfusH = 6.03 KJ mol-1 at 0°C.
Cp [H2O(l)] = 75.3 J mol-1 K-1;
Cp [H2O(s)] = 36.8 J mol-1 K-1.
10. Calculate the enthalpy change on freezing of 1.0 mol of water at 10.0°C to ice at -10.0°C. A, ΔfusH = 6.03 KJ mol-1 at 0°C.
Cp [H2O(l)] = 75.3 J mol-1 K-1;
Cp [H2O(s)] = 36.8 J mol-1 K-1.
-
1 Answer
-
10. Total enthalpy change involved in the transformation is the sum of the following changes:
(i) Energy change involved in the transformation of 1 mol of water at 10°C to 1 mol of water at 0°C
ΔH= Cp [H2O (l)] ΔT
(ii) Energy change involved in the transformation of 1 mol of water at 10°C to 1 mol of ice at 0°C
ΔHfreezing
(iii) Energy change involved in the transformation of 1 mol of ice at 10°C to 1 mol of ice at 0°C
&n
...more
Similar Questions for you
Kindly go through the solution
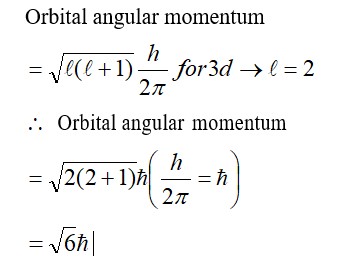
(1) [Ni (NH3)6]+2 → Ni+2 → d8, C. No. = 6,
SP3d2, Para
(2) [Co (H2O)6]+2 → Co+2 → d6, C. No. = 6
d2sp3, Dia
(3) [Ti (H2O)6]+3 → Ti+3 → d1, C. No. = 6
d2SP3, Para
(4) [Co (NH3)6]+3 → Co+3 → d5, C. No. = 6
d2sp3, Para
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else
Sign Up on ShikshaOn Shiksha, get access to
- 66k Colleges
- 1.2k Exams
- 681k Reviews
- 1800k Answers