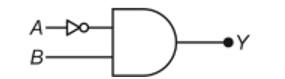
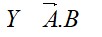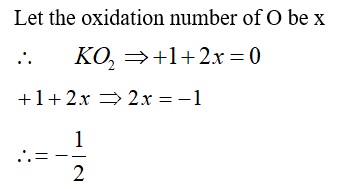11.12. Explain the difference in properties of diamond and graphite on the basis of their structures.
11.12. Explain the difference in properties of diamond and graphite on the basis of their structures.
2 Views|Posted 9 months ago
Asked by Shiksha User
1 Answer
V
Answered by
9 months ago
Diamond has a crystalline lattice where each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three- dimensional network of carbon ato
Similar Questions for you
From BF3 to BI3 Lewis acidic strength increases
F2 is the strongest oxidising agent
HClO4 is the most acidic compound.
Taking an Exam? Selecting a College?
Get authentic answers from experts, students and alumni that you won't find anywhere else.
On Shiksha, get access to
66K
Colleges
|
1.2K
Exams
|
6.9L
Reviews
|
1.8M
Answers
Learn more about...
Didn't find the answer you were looking for?
Search from Shiksha's 1 lakh+ Topics
or
Ask Current Students, Alumni & our Experts
Have a question related to your career & education?
or
See what others like you are asking & answering